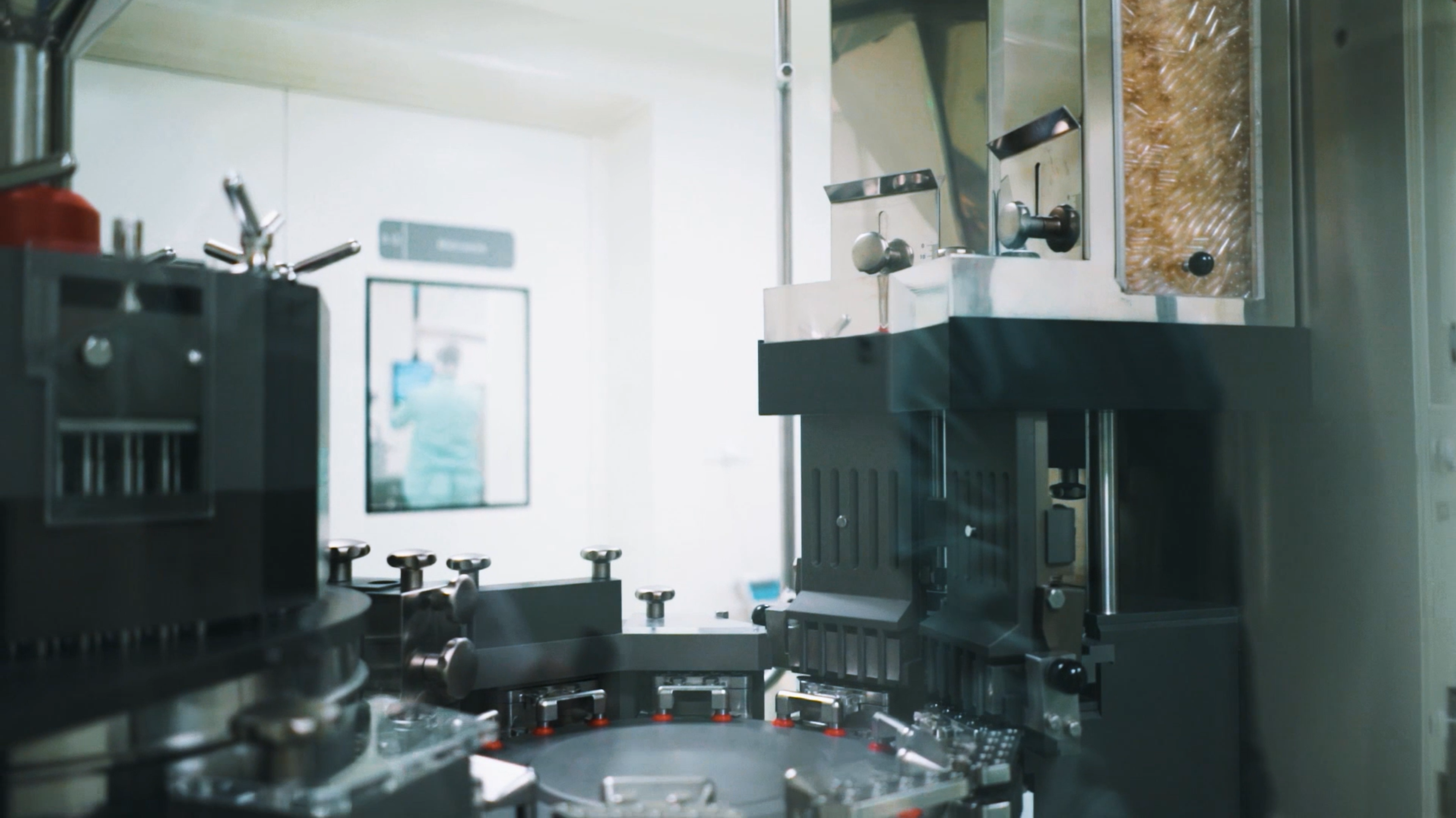
SANPROBI's production facility is the most advanced probiotic packaging plant in Europe.
It is registered as a food manufacturer and operates in accordance with the HACCP food safety system. In fact, it has been designed and built in accordance with pharmaceutical manufacturing standards (i.e. GMP requirements).

Production has been planned and organised to minimise the impact of factors that adversely affect the stability and quality of the bacterial strains contained in the final products, i.e. humidity and temperature.
The plant also pays particular attention to protecting the finished products from contamination, including cross-contamination, using equipment and procedures that ensure pharmaceutical grade cleanliness (pharmaceutical class D). HEPA 13 filters are used, as well as a pressure cascade between rooms to prevent the movement of probiotic dust.


Consists of a circulation corridor, social room, ‘dirty’ changing rooms, toilets, waste, capsule, film, packaging, final product and reject product storage, receiving room, cold room, probiotic ripening room, utility room and dispatch room.

Consists of ‘clean’ changing rooms, a personnel and material airlock, a packing room and a sanitary room.

It consists of a personnel and material airlock, a traffic corridor, a washing room, a capsule and blister packing room and a detergent storage room.
An extensive ventilation and air conditioning system is used throughout the facility to control temperature and humidity parameters, as well as other special solutions.

The temperature throughout the clean zone is maintained at 20°C with a maximum variation of +/- 5°C.

A pressure cascade has been installed throughout the clean zone to eliminate contamination, in particular the transfer of probiotic dust between rooms.

The humidity in the clean zone is kept below 40%. This is undoubtedly stricter than usual to protect the probiotic bacteria, which are sensitive to humidity.

HEPA 13 filters are used throughout the clean zone to maintain air purity as in Class D pharmaceutical plants.

The air temperature in the cold store is maintained at 2-8°C.

All parameters of the ventilation and air conditioning system are constantly monitored electronically.
SANPROBI’s manufacturing facility is equipped with state-of-the-art machinery from leading manufacturers of equipment for the pharmaceutical industry.
Moving from the grey zone to the clean zone is subject to a protective procedure and requires two changes of protective clothing, a change of shoes, the use of hair caps, protective masks, and hand washing and disinfection each time.
Only persons authorised by the plant manager, who will appoint an escort working in the plant area, may enter the clean zone. This designated person is responsible for the safety of visitors and for training them in the rules of conduct and the use of protective clothing.
Only persons with up-to-date sanitary and epidemiological tests are allowed to enter the production areas of the clean zone. For visitors, guests and technical staff, a signed health and safety statement will be accepted in the absence of a health certificate. The statement is valid for 3 months from the date of signature.